

All Genoss products are manufactured in accordance with a systematic
and rigorous quality control system based on ISO standards.

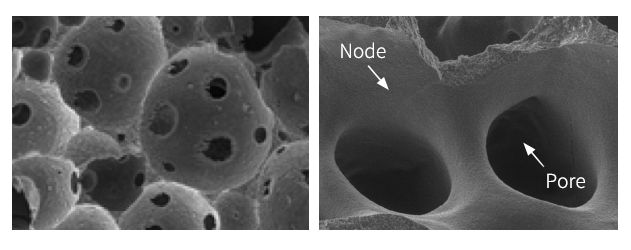

| Name | Function |
| Pore | Space for cell growth and blood vessel |
| Node | Main support (scaffold) |
| Product | Shape | Model Name | Size(mm) | Capacity(cc) |
| Granule (Vial package) |
 |
3G0205010 3G0205025 3G0205050 3G0205100 3G0205200 |
0.2 ~ 0.5 |
0.10 0.25 0.5 1.0 2.0 |
|
3G0210010 3G0210025 3G0210050 3G0210100 3G0210200 |
0.2 ~ 1.0 |
0.10 0.25 0.5 1.0 2.0 |
||
|
3G0510010 3G0510025 3G0510050 3G0510100 3G0510200 |
0.5 ~ 1.0 |
0.10 0.25 0.5 1.0 2.0 |
||
|
3G1020010 3G1020025 3G1020050 3G1020100 3G1020200 |
1.0 ~ 2.0 |
0.10 0.25 0.5 1.0 2.0 |
||
| Block Type |  |
3B050510 3B050530 3B051010 3B051015 3B080810 |
05x05x10 05x05x30 05x10x10 05x10x15 08x08x10 |
0.25 0.75 0.5 0.75 0.64 |
| Granule (Φ5.0mm Syringe package) |
 |
3G0205025L 3G0205050L |
0.2 ~ 0.5 |
0.25 0.5 |
|
3G0210025L 3G0210050L |
0.2 ~ 1.0 |
0.25 0.5 |
||
|
3G0510025L 3G0510050L |
0.5 ~ 1.0 |
0.25 0.5 |
||
|
3G1020025L 3G1020050L |
1.0 ~ 2.0 |
0.25 0.5 |
||
| Granule (Φ7.0mm Syringe package) |
 |
3G0205025S 3G0205050S |
0.2 ~ 0.5 |
0.25 0.5 |
|
3G0210025S 3G0210050S |
0.2 ~ 1.0 |
0.25 0.5 |
||
|
3G0510025S 3G0510050S |
0.5 ~ 1.0 |
0.25 0.5 |
||
|
3G1020025S 3G1020050S |
1.0 ~ 2.0 |
0.25 0.5 |
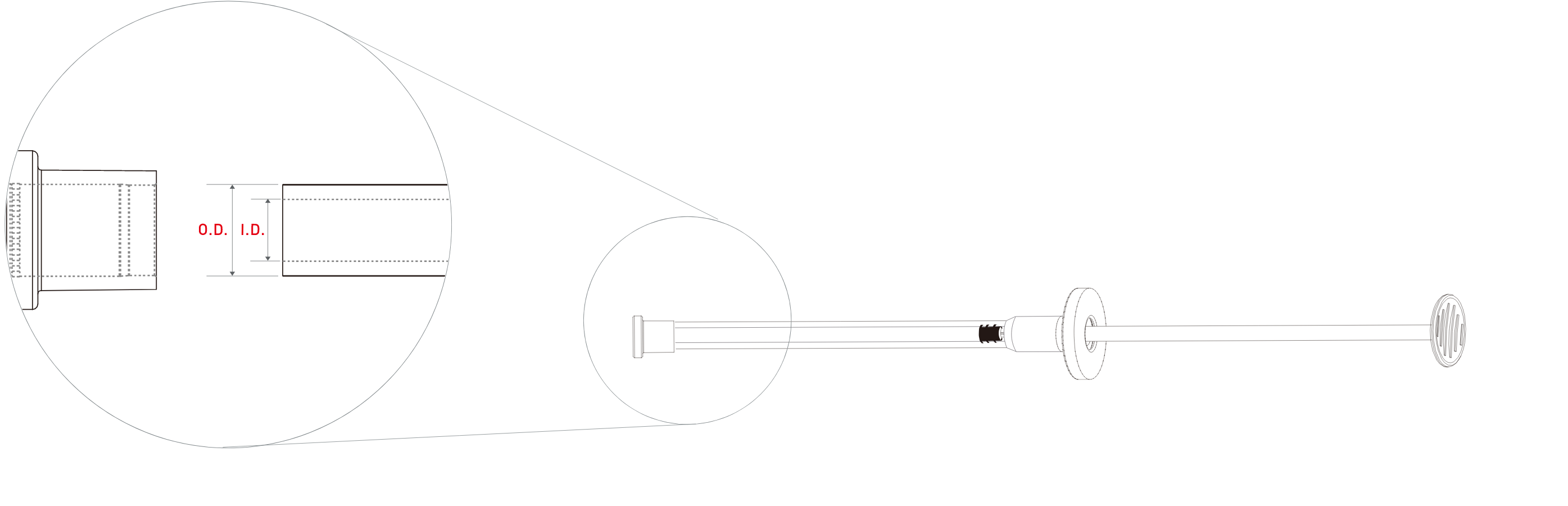

| Product | External Diameter(mm) | Internal Diameter(mm) |
| OSTEON 3 Sinus | ϕ7.0 | ϕ5.0 |
| OSTEON 3 Lifting | ϕ5.0 | ϕ3.4 |

