Applications
- Alveolar bone augmentation
- Filling of lost areas around implants
- Filling of tooth extraction area to protect alveolar bone
- Maxillary sinus lift surgery
- Filling of periodontal defects
Characteristic
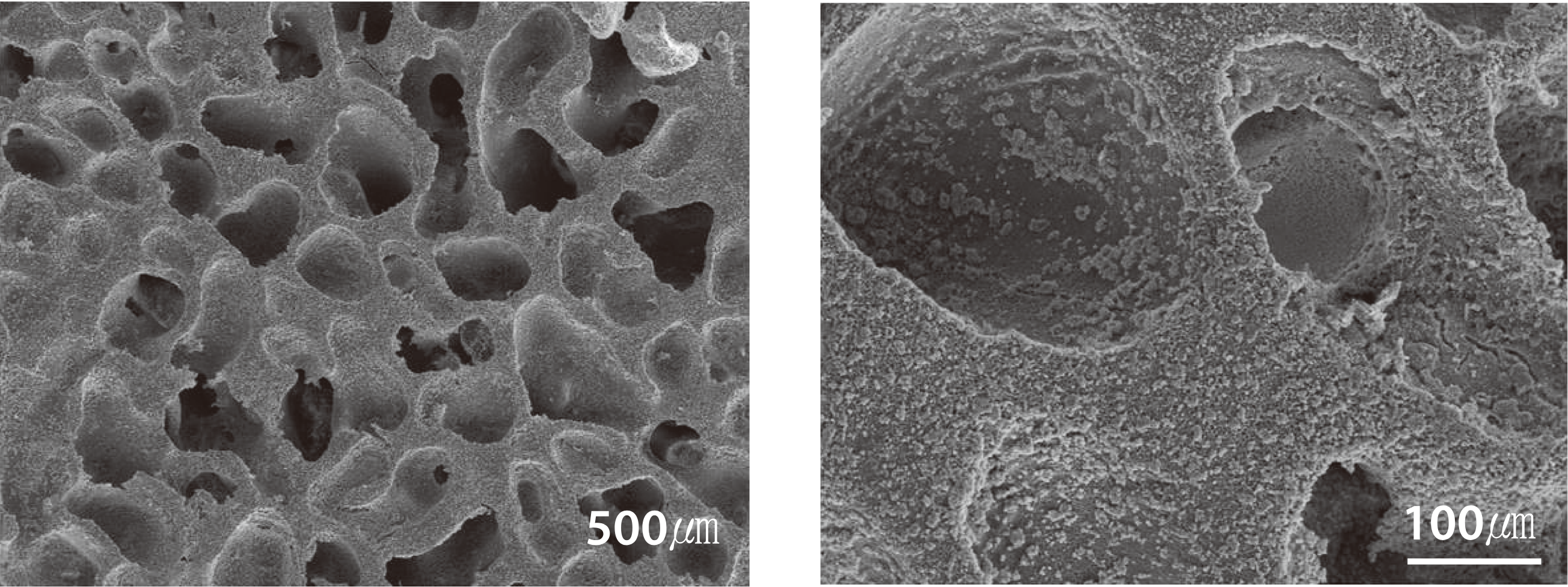
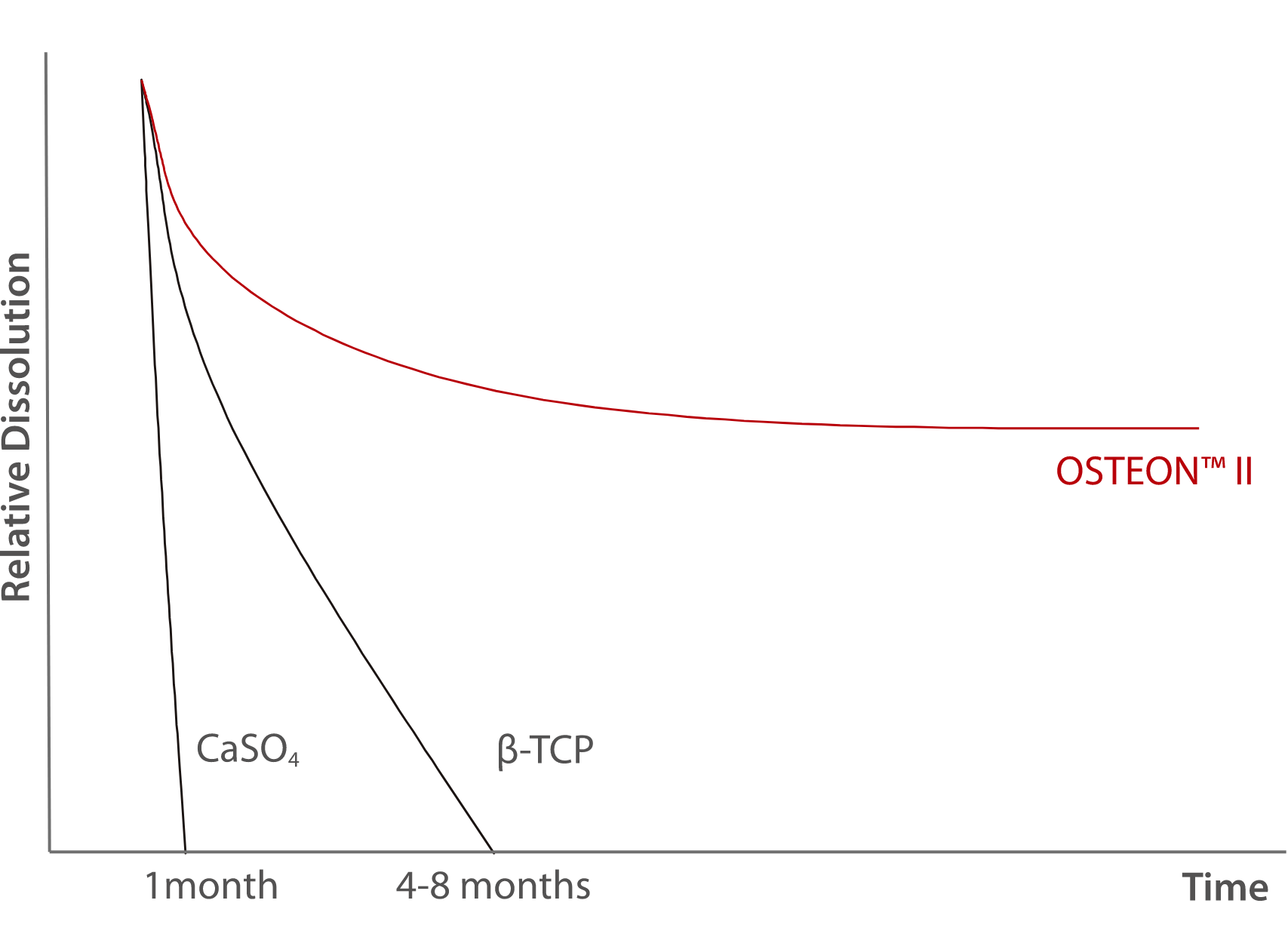
- Osteoconductive synthetic bone graft material
- Pore size: approximately 250㎛ (Micro CT measurement)
- Porosity: more than 70%
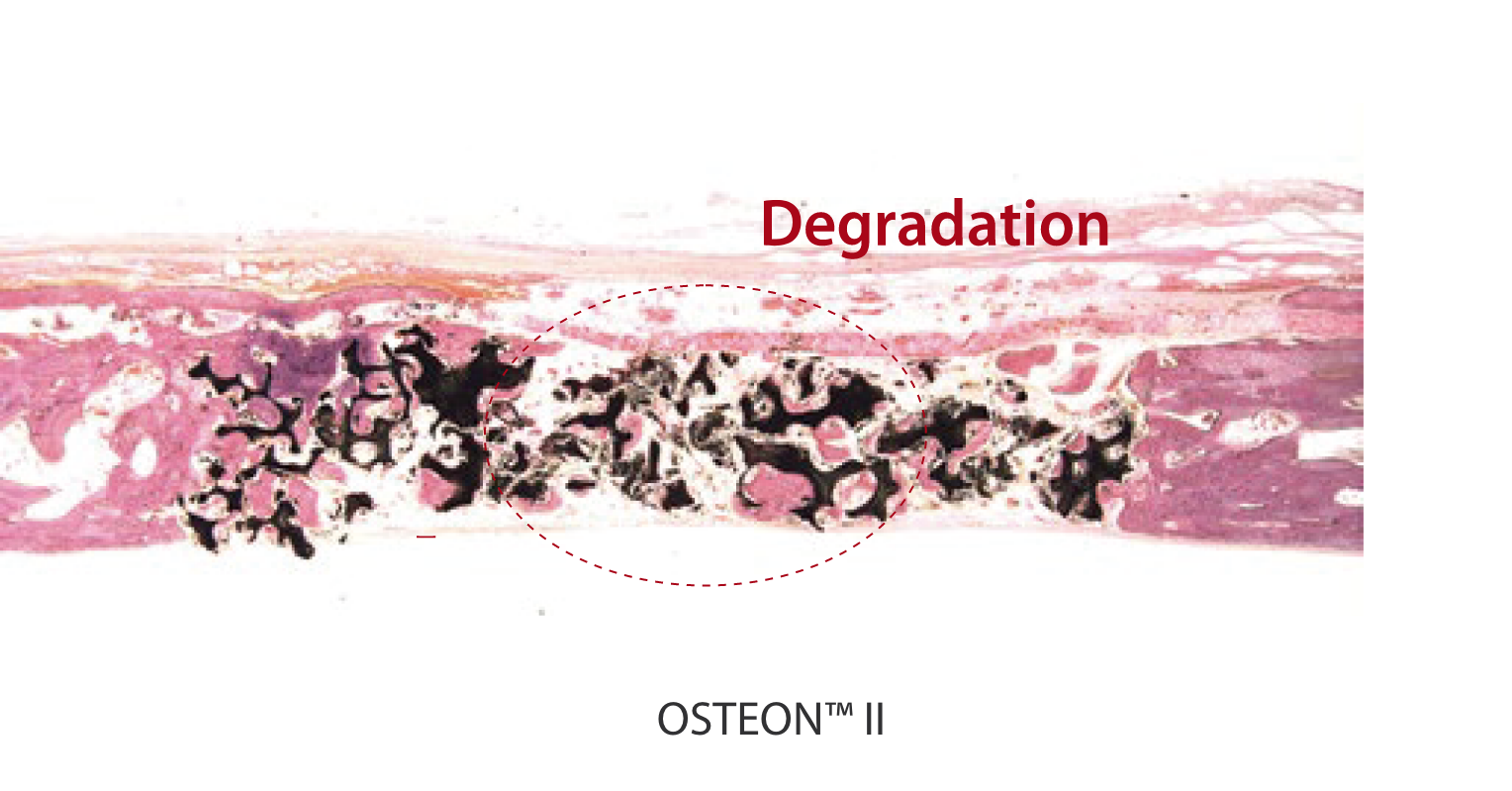
Animal Test Result
12-weeks follow up in rabbit calvaria model
Product Specification
| Type |
Model Name |
Grain Size(mm) |
Capacity(cc) |
| Vial |
DT7G0205 |
0.2 ~ 0.5 |
0.1 / 0.25 /0.5 / 1.0 /2.0 |
| DT7G0510 |
0.5 ~ 1.0 |
| DT7G1020 |
1.0 ~ 2.0 |
| Sinus(Syringe) |
DT7G0510050SS |
0.5 ~ 1.0 |
0.5 |
| DT7G0520050SS |
1.0 ~ 2.0 |
| Lifting(Syringe) |
DT7G0205025LS |
0.2 ~ 0.5 |
0.25 |
| DT7G0510025LS |
0.5 ~ 1.0 |
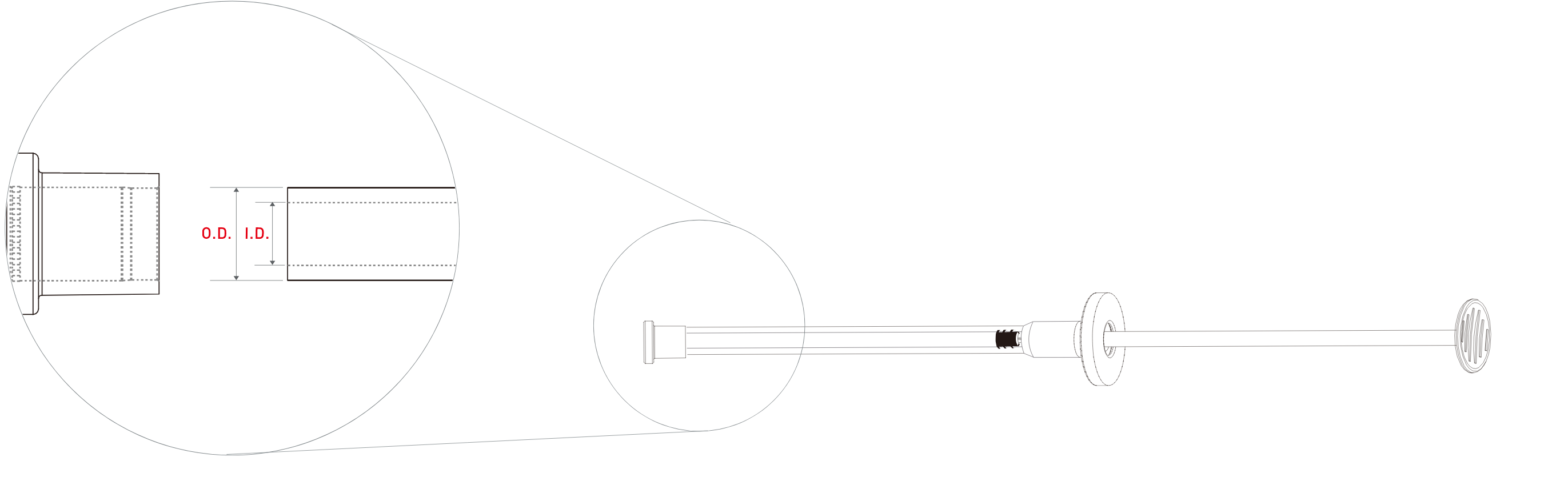
Syringe
| Product |
External Diameter(mm) |
Internal Diameter(mm) |
| OSTEON II Sinus |
ϕ7.0 |
ϕ5.0 |
| OSTEON II Lifting |
ϕ5.0 |
ϕ3.4 |
Instruction for OSTEON II Sinus & Lifting